Overview
MATRIX: A USAID Project to Advance the Research and Development of Innovative HIV Prevention Products for Women
MATRIX is a five-year program funded by the U.S. Agency for International Development (USAID) in 2021 with a mission to expedite the research and development of HIV prevention products for women – including products designed to protect against both HIV and pregnancy – that in addition to being safe and effective, will be acceptable, affordable, scalable and deliverable in the settings where they are needed most.
MATRIX activities are focused on the early research and development of products, which involves both pre-clinical research – the laboratory and animal studies needed to support a product’s evaluation in humans – and the first clinical trials of products. Through its North-South Partnerships, MATRIX seeks also to strengthen the research and development capacity of African investigators in order to facilitate full and sustainable ownership of this work. MATRIX stands for Microbicide R&D to Advance HIV Prevention Technologies through Responsive Innovation and eXcellence.
MATRIX is being implemented by Magee-Womens Research Institute (MWRI) in collaboration with nearly 20 partner organizations based in Kenya, South Africa, the United States and Zimbabwe. Leading the project is Sharon Hillier, Ph.D., of MWRI and the University of Pittsburgh, USA, with Thesla Palanee-Phillips, Ph.D., from the Wits Reproductive Health and HIV Institute (Wits RHI) and University of Witwatersrand, South Africa, serving as deputy director. Collectively, MATRIX partners have expertise across multiple fields, including drug formulation, drug delivery and product development; clinical trials design and implementation; human-centered design and socio-behavioral research; market strategy and business case development; capacity strengthening; and stakeholder engagement.
How is MATRIX unique?
The research and development of new products begins with rigorous testing in the laboratory and then in animals that seek to demonstrate their safety and efficacy. These studies must be conducted, and other regulatory requirements be met, before a product can proceed to human clinical trials. The products being developed under MATRIX must also meet a second set of standards that considers what women want and are likely to use and whether the product will be practical and feasible to introduce in countries most affected by HIV. Indeed, a key feature of MATRIX is its focus on being responsive to end-user and stakeholder feedback during the earliest stages of product development to inform decisions about product design and MATRIX’s overall research agenda.
While early-phase clinical trials of new HIV prevention products have typically been conducted in the United States or Europe, MATRIX is also conducting these kinds of studies in sub-Saharan Africa in order to gain important insight into the safety and acceptability of new products in the populations that are most important.
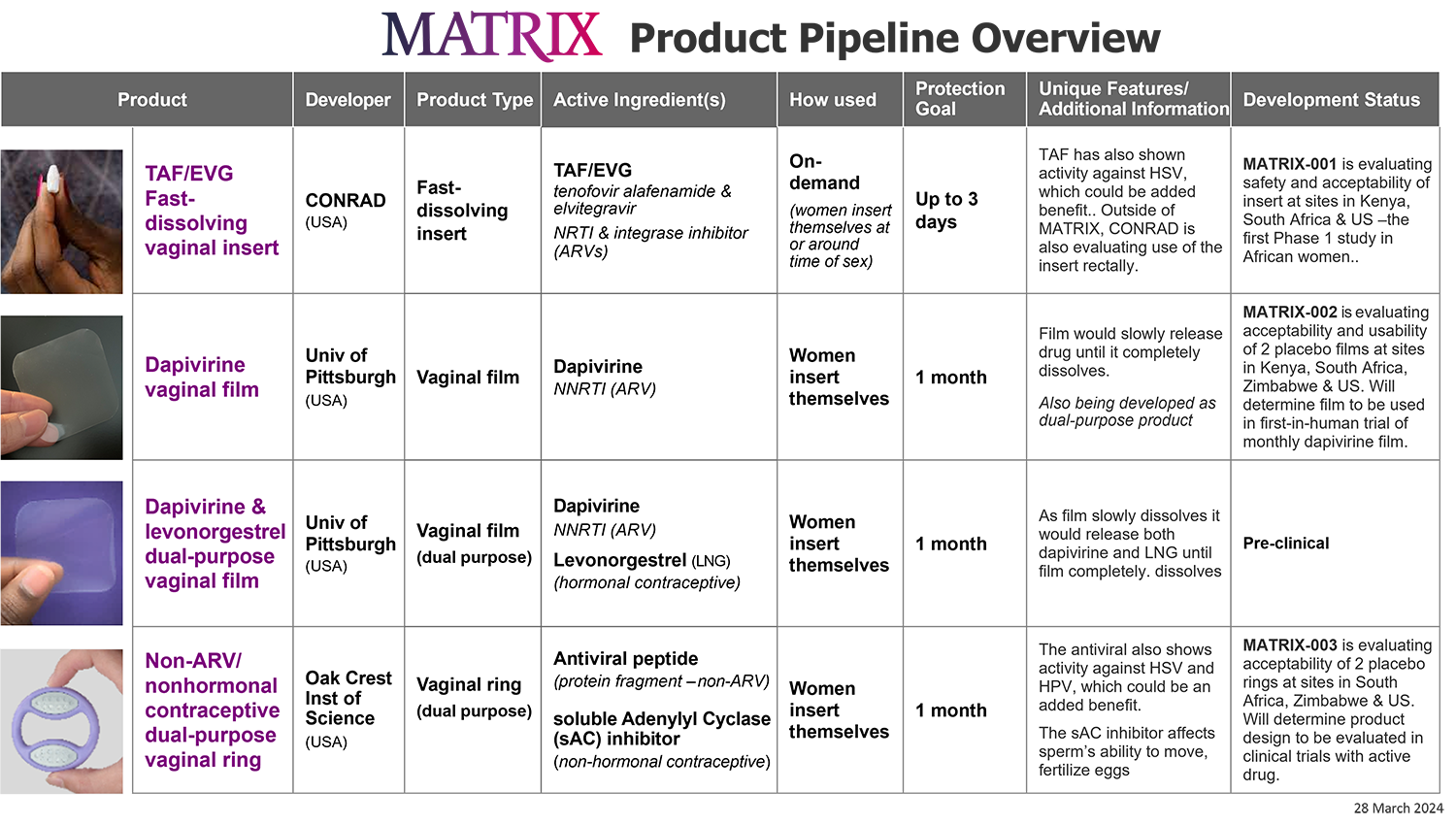
The MATRIX Product Pipeline
MATRIX’s primary focus is on the development of HIV prevention products for women, including HIV prevention products that provide contraception. So that women can have different options, we seek diversity in our products – from vaginal products that are short-acting and to be used at the time of sex, to those used for a month at a time and systemic products that could provide protection for up to a year.
Early research and development is unpredictable – a product that initially appears promising can face insurmountable challenges at any point along the way. MATRIX takes an approach that sets clear milestones and benchmarks for its products and can respond quickly to technical hurdles and/or developments within the field to prioritize those products that have the greatest chance for success and would add value. As such, the MATRIX pipeline of products is dynamic. In 2022, MATRIX was supporting six products, then the product pipeline expanded to nine products in 2023. Our current portfolio includes four products, and this, too, could change with the addition of new products and/or should further refinements be necessary.
How MATRIX is structured
MATRIX consists of five activity hubs:
Technology Accelerator manages the development process of the MATRIX product pipeline, and with the input of an independent Scientific Advisory Group, advises on a product’s next steps, i.e., whether it can proceed to a placebo study or Phase 1 clinical study, whether additional animal and laboratory studies and/or product design modifications are needed; or whether to stop its development. The Technology Accelerator also provides support to other research and development endeavors through seed funding and other grants, including of projects led by African investigators.
Clinical Trials oversees the design and implementation of placebo studies and Phase 1 studies of products at MATRIX partner clinical trial sites in the U.S., Kenya, South Africa and Zimbabwe.
Design to Delivery (D2D) includes two components, one that conducts end-user research to understand women’s preferences for different MATRIX products and product attributes; and a second that designs and implements behavioral studies and socio-behavioral research within clinical trials.
Business, Market Dynamics and Commercialization (BACH) conducts business case and market analysis as well as seeks linkages with possible investors.
Capacity Strengthening, Engagement and Mentorship (CaSE) matches African investigators with mentorship and fellowship opportunities, with an emphasis on early research and development.
| Description | Date | Size |
|---|
| About MATRIX Factsheet (Download) | 2024-07-01 | 703.59 KB |
| MATRIX Product Pipeline (PDF) (Download) | 2024-03-28 | 183.18 KB |
