TAF/EVG Fast Dissolving Vaginal Insert
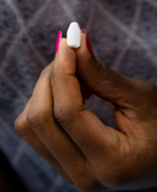
The TAF/EVG fast-dissolving vaginal insert is an on-demand HIV prevention product that women would insert into their vagina around the time of sex. The insert, which resembles a bullet-shaped tablet, contains the antiretroviral (ARV) drugs tenofovir alafenamide (TAF) and elvitegravir (EVG). Once inside the vagina, the insert is designed to rapidly dissolve, and in doing so, release both TAF and EVG which, by mixing with vaginal fluid, get dispersed inside the vagina and into the tissue. Based on animal and clinical laboratory studies, a single vaginal dose of the TAF/EVG insert is expected to provide HIV protection for at least a day, regardless of the number of sex acts and even if used within a few hours after sex.
The insert is being developed by CONRAD, a nonprofit research organization affiliated with Eastern Virginia Medical School in Norfolk, Va., for its use both vaginally and rectally. TAF and EVG are being provided by Gilead Sciences for CONRAD’s development of the insert product.
TAF belongs to a class of ARVs called nucleoside reverse transcriptase inhibitors (NRTIs) that prevent HIV from making copies of itself inside human cells, thereby preventing the spread of HIV inside the body. TAF is approved by the U.S. Food and Drug Administration (FDA) for the treatment and prevention of HIV when used daily in combination with other ARV drugs. Laboratory and animal studies also indicate that TAF has activity against herpes simplex virus (HSV). The insert’s second drug, EVG, is FDA-approved for the treatment of HIV in combination with other ARVs. EVG is an integrase inhibitor that blocks HIV from being able to integrate its genetic code into human cells – a step that occurs later in the HIV lifecycle. The two drugs act synergistically to block HIV infection.
MATRIX is evaluating the insert for its primary indication as a product to prevent HIV and as a vaginal insert. Toward this end, it is conducting MATRIX-001, which is the second Phase 1 trial of the TAF/EVG insert used vaginally and the first to enroll African women. The insert is the only female-controlled on-demand method currently in clinical trials.
The MATRIX-001 study is evaluating the safety of the insert when used multiple times over several days, as well as user acceptability and how and where the two drugs are taken up in the body. The study will help determine whether the product should advance to Phase 2 studies to assess its safety and proof-of-concept effectiveness when used as prescribed, i.e., at or around the time of sex.
As an on-demand method, the insert could appeal to women who have infrequent or clustered sex and want to use a product only when needed and because it delivers drug locally (in the vagina), with little drug going elsewhere in the body. It would provide an alternative for women who don’t want to use systemic products, such as oral pre-exposure prophylaxis (PrEP), which requires taking an ARV tablet every day, or long-acting products like the monthly dapivirine vaginal ring or cabotegravir injections given every two months.
MATRIX News Releases
“On-demand” HIV prevention method for women being tested in second early phase trial
QA
The TAF/EVG Fast-Dissolving Vaginal Insert and MATRIX-001 Phase 1 Study
